This chapter should be cited as follows:
Val Id, Aidé S, et al, Glob. libr. women's med.,
ISSN: 1756-2228; DOI 10.3843/GLOWM.419953
The Continuous Textbook of Women’s Medicine Series – Gynecology Module
Volume 12
Infections in gynecology
Volume Editors:
Professor Francesco De Seta, Department of Medical, Surgical and Health Sciences, Institute for Maternal and Child Health, University of Trieste, IRCCS Burlo Garofolo, Trieste, Italy
Dr Pedro Vieira Baptista, Lower Genital Tract Unit, Centro Hospitalar de São João and Department of Gynecology-Obstetrics and Pediatrics, Faculdade de Medicina da Universidade do Porto, Portugal

Chapter
Genital Herpes
First published: August 2023
Study Assessment Option
By completing 4 multiple-choice questions (randomly selected) after studying this chapter readers can qualify for Continuing Professional Development awards from FIGO plus a Study Completion Certificate from GLOWM
See end of chapter for details
INTRODUCTION
Genital herpes simplex virus (HSV) infections are a major global public health problem. HSV establishes a latent state, which may or may not be followed by locally recurrent viral reactivation, which depending on the frequency and severity of recurrences in a year, may compromise the personal, social, and sexual quality of life.
Perinatal transmission of HSV can lead to significant fetal morbidity and mortality. In addition, there is a link between HSV-related genital ulceration and sexual transmission of human immunodeficiency virus (HIV).
DEFINITION
Genital herpes is a chronic, lifelong viral infection. Herpes simplex viruses (HSV) are double-stranded DNA-enveloped viruses. They belong to the Herpesviridae family. There are two distinct serotypes, herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2).1
Due to tropism for anatomical sites, HSV-1 predominantly infects the mucous membranes and skin of the orolabial region. However, HSV-1 is now being seen more frequently in genitalia due to the decreased prevalence of orolabial HSV-1 in the young adult population and the increased frequency of oral sex. HSV-2 usually infects the genitalia. Despite this site tropism, both genotypes can be isolated from the orolabial and genital regions.1
EPIDEMIOLOGY
HSV-1 and -2 are common infections worldwide. Both can cause genital herpes. infection of the same anatomical site can be caused by both. However, genital HSV is often under-recognized because the infection is often subclinical. Most people infected with HSV-2 have not had the condition diagnosed, many of whom have mild symptoms or unrecognized infections, but intermittently excrete virus in the anogenital area. Consequently, most genital herpes infections are transmitted by people who are not aware that they are infected or who are asymptomatic when transmission occurs.1,2
The prevalence of HSV-2 has decreased over time in the United States among patients between 14 and 49 years old. From 1999 to 2000, HSV-2 seroprevalence was 18%. From 2005 to 2010, it was approximately 16%. And during 2015–2016, it dropped to 11.9%, while HSV-1 seroprevalence was 47.8%.3,4
HSV-2 seroprevalence seems to increase with age (onset of sexual activity) and number of sexual partnerships, and is higher among women compared to men (21% versus 12%). Also, it is higher among non-Hispanic black patients (39%) than in non-Hispanic white patients (12%). It varies by population and according to high-risk behavior, with the worldwide prevalence ranging between 10% and 40%. It is also higher in people living with HIV compared to the general population.1,3,5
An increasing proportion of anogenital herpetic infections have been attributed to HSV-1, which is especially prominent among young women and in men who have sex with men (MSM). Previous HSV-1 infection does not appear to affect the rate of HSV-2 acquisition.5
The frequency of recurrence depends on the severity and duration of the initial episode, the infecting serotype, and the host. Most cases of recurrent genital herpes are caused by HSV-2.1,5In a prospective study of 137 patients with a symptomatic first episode of genital herpes, the probability of recurrence was higher in those with HSV-2 infections (60% versus 14% in those with HSV-1).6
PHYSIOPATHOLOGY
HSV normally infects epithelial cells of the skin and mucosa through small breaks and then travels by retrograde transport along the sensory nerves to the sensory root ganglia, where viral replication occurs. The establishment of latency occurs throughout the life of the host. Periodically there may be a reactivation of the latent state, with anterograde transfer from the sensory ganglia to the periphery (skin or mucous membranes), at which time there is subclinical excretion or overt symptoms.1,3
Transmission occurs not only by penetrative intercourse but also by genital skin-to-skin contact with infected partners. These infected partners are excreting the virus during a clinically evident but not necessarily symptomatic herpetic episode (clinical reactivation), or more commonly during an episode of subclinical or asymptomatic excretion.1,3
In the areas of viral reactivation and excretion, it is evident that excretion is eliminated by immune responses, as measured by local CD8 memory cells. Thus, there is a chronic local immune response, which contributes in part to the increased susceptibility to HIV infection (approximately 2–3 times higher).1,3
RISK FACTORS
Potential risk factors for acquiring genital herpes include biological and behavioral components. HSV-2 infection is more commonly seen in females, and in individuals with a history of sexually transmitted infections (STIs), early sexual debut with increasing numbers of sexual partners, sexual contact with sex workers, viral shedding in known positive partners when the other partner is unsure of his/her status, and unprotected sex. According to data pooled from six studies, there was a 3.6% increase in the odds of HSV-2 acquisition with each unprotected act of intercourse, but no increase in the odds of acquisition associated with protected acts.7 Antiviral therapy reduces the frequency of HSV reactivation. However, it is extremely important to promote educational measures and to recommend the detection of HSV-2 through type-specific serological tests, as clinical history and the absence of genital lesions do not exclude the presence of herpetic infection.8
CLINICAL MANIFESTATIONS
The clinical manifestations of genital herpes simplex virus vary widely depending on whether the infection is primary, non-primary, or recurrent. Due to the host immune response, the HSV lesions are self-limited.3,5
Primary infection – refers to infection in a patient with no pre-existing antibodies to HSV-1 or HSV-2.
Non-primary infection – non-primary first episode infection refers to the acquisition of genital HSV-1 in a patient with pre-existing antibodies to HSV-2 or the acquisition of genital HSV-2 in a patient with pre-existing antibodies to HSV-1.
Recurrent episode – recurrent episode refers to the reactivation of genital HSV where the type of HSV recovered in the lesion is the same type as the antibodies in the serum.
Each of these types can be symptomatic or asymptomatic (also called subclinical). Asymptomatic infection will only be detected if the patient is tested by culture or polymerase chain reaction (PCR).
In primary infection, the average incubation period for the development of genital herpes after exposure is 4 days (with a range of 2 to 12 days). The clinical manifestations of primary genital HSV infection are highly variable. The initial presentation may be severe with painful genital ulcers (Figure 1) and may be accompanied by dysuria, fever, myalgias, malaise, local painful inguinal lymphadenopathy, and headache. However, in other patients, the infection may be mild, subclinical, or completely asymptomatic. There are no clear differences in clinical presentation based on the infecting virus (i.e., HSV-1 versus HSV-2). Primary genital herpes is commonly associated with viremia.3,5
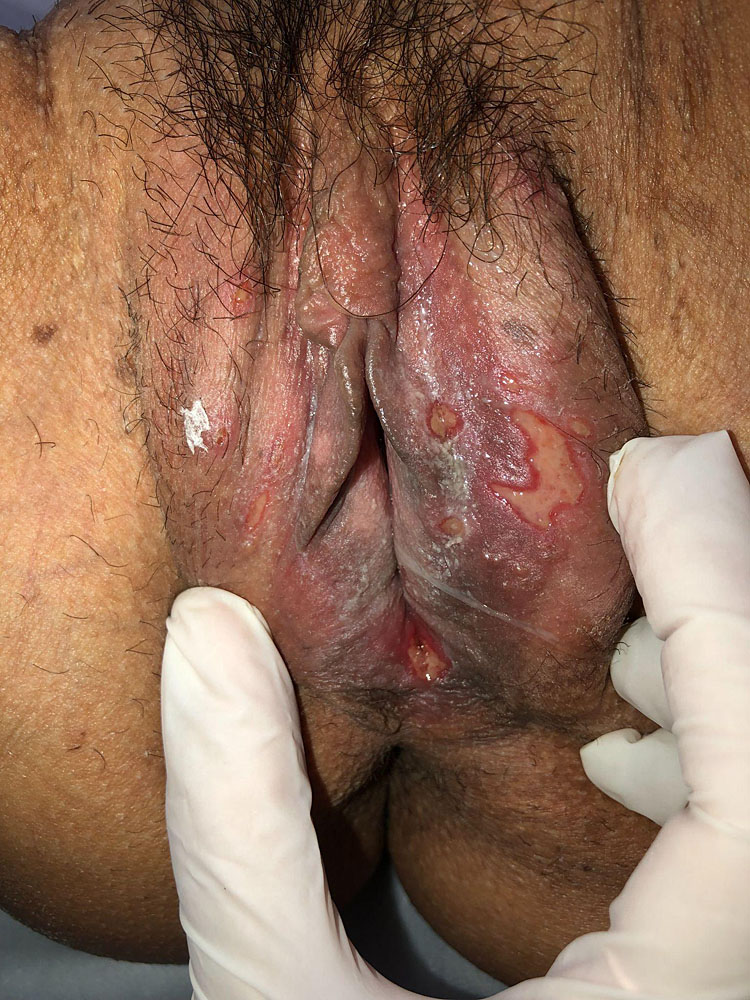
1
Initial more severe presentation with painful genital ulcers and painful local inguinal lymphadenopathy.
The characteristic skin lesions of HSV infection begin as clustered, 2 to 4 mm, multiple, bilateral vesicles with associated underlying erythema, which evolve into vesicopustules, and which in turn develop erosions and ulcerations (Figure 2). Symptoms tend to be more severe in women than in men.3,5
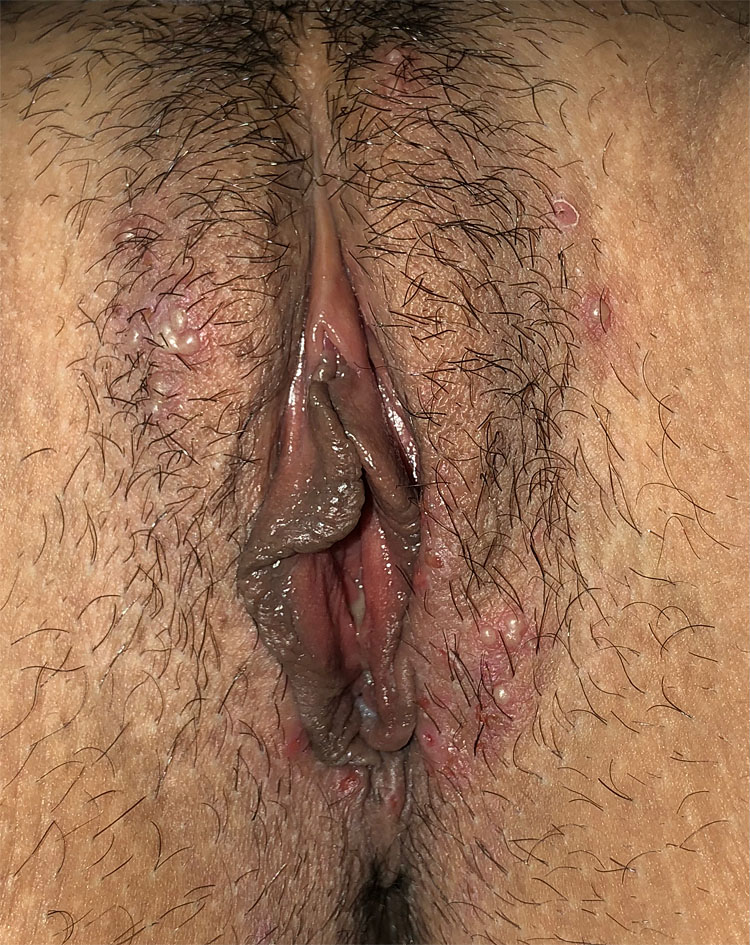
2
Clustered 2–4 mm, multiple, bilateral vesicles with associated underlying erythema, evolving into vesicopustules and these with erosions and ulcerations.
Dysuria can lead to reluctancy to urinate due to the passage of urine in open, inflamed vesicles, leading to acute urinary retention; this can be managed with sitz baths. However, acute urinary retention due to loss of sacral sensation can occur due to lumbosacral radiculomyelitis secondary to primary severe HSV infection.1,3
Primary genital HSV-2 infections have been associated with more frequent and prolonged asymptomatic viral excretion from the genitourinary tract compared to HSV-1 infections.1,3
The first episode of non-primary infection is associated with fewer lesions and fewer systemic symptoms than primary infection, presumably because antibodies against one type of HSV confer partial protection against the other.1,3
Clinical recurrences of genital HSV are common but are generally less severe than primary or non-primary infections (Figure 3). The mean duration of lesions is generally shorter in recurrences than in primary infection (10 versus 19 days) and the duration of viral excretion is usually of 2 to 5 days.1,3
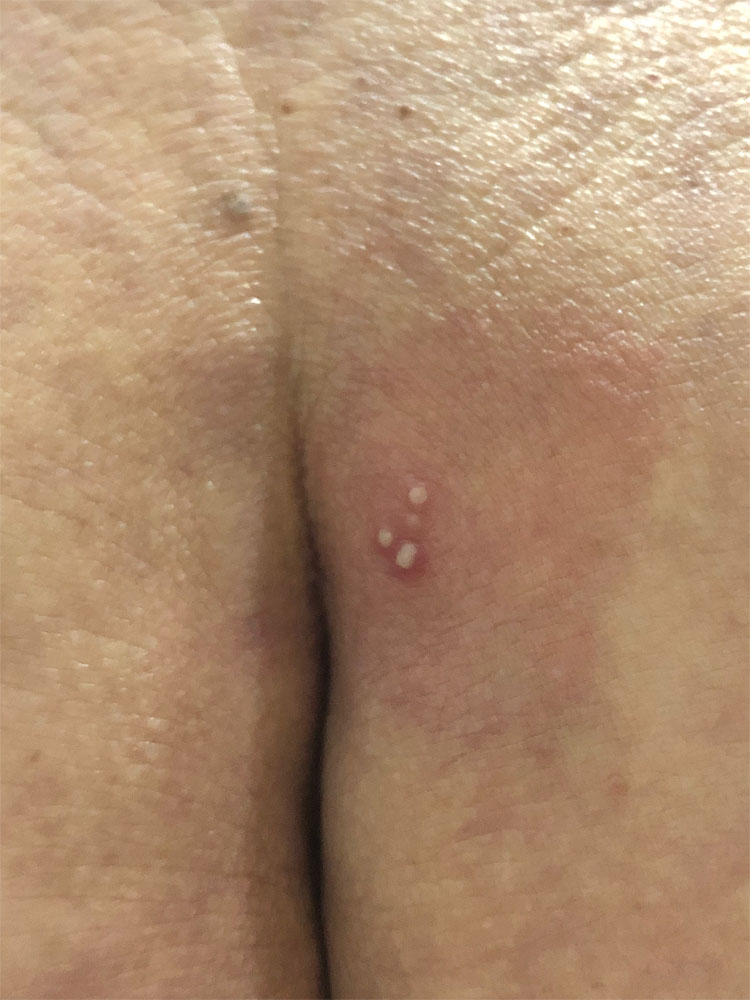
3
Clinical recurrences of genital HSV are less severe, usually occurring at the same site.
In recurrent infection, lesions are typically described as small, unilateral vesicular or ulcerative lesions. Atypical presentation lesions include fissures or vulvar irritation. Systemic symptoms are infrequent, and approximately 25% of recurrent episodes are completely asymptomatic.
About 50% of patients with symptomatic recurrences have prodromal symptoms before the eruption, such as mild local tingling or sharp pain in the buttocks, legs, and hips.1,3
Extragenital complications (radiculitis, aseptic meningitis – 8%, urinary retention due to sacral autonomic nervous system dysfunction from sacral radiculitis – 2%, and proctitis, particularly in MSM) occur in a minority of patients presenting with primary herpes simplex virus infection but may recur with subsequent episodes.1,3
DIAGNOSIS
Clinical
Herpes simplex virus infection is the second most common STI worldwide and is the most common cause of genital ulcers in the developed world. As a result of the wide variation in manifestations of genital herpes, purely clinical diagnoses have been shown to have low sensitivity, making laboratory tests necessary for diagnostic confirmation.2,3,5
Laboratory
It is advisable to distinguish between HSV-1 and HSV-2 as it correlates with the prognosis and future counseling.
According to the Sexually Transmitted Infections Treatment Guidelines, 2021,5 type-specific virology and type-specific serology tests should be used. HSV-2 genital herpes infection increases the risk of contracting HIV by two to three times. Therefore, people with or at risk for STIs should be tested for genital herpes.
Cases of recurrence and viral shedding in the absence of genital lesions are more frequent in cases of HSV-2 compared to HSV-1. When we are faced with individual presenting lesions characteristic of herpes, type-specific virologic testing from the lesion by nucleic acid amplification test (NAAT) including PCR, or culture must be performed. In the absence of lesions, a type-specific serology test is recommended. However, type-specific HSV-2 serologic assays for diagnosing HSV-2 are useful in case of recurrence, atypical lesions with a negative HSV PCR or culture result, clinical diagnosis of genital herpes without laboratory confirmation, and when the current partner has genital herpes. Otherwise, it is not recommended among the general population.5
The serological exam can be performed in the first weeks after infection, remaining positive indefinitely. The sensitivity of type-specific tests for detecting HSV-2 antibodies varies from 80% to 98%. If the result comes back negative, the exam must be repeated in 12 weeks.3,5
HSV PCR of blood should only be requested in the case of disseminated infection (e.g., hepatitis), but is not indicated for the diagnosis of genital herpes.5
Differential diagnosis
Most genital ulcers are caused by HSV. Among patients presenting with genital ulcers, the primary differential diagnosis includes infectious and non-infectious diseases. Among the infectious diseases are those that are sexually transmitted (STI) and non-sexually transmitted. The STIs included in the differential diagnosis include: syphilis and chancroid, as well as donovanosis and lymphogranuloma venereum. Drug eruptions, autoimmune diseases such as Crohn's disease, Behcet's syndrome, erosive lichen planus, and lichen sclerosus are among the non-infectious causes. Additionally, there are Lipschutz ulcers (Figure 4), as well as ulcers resulting from trauma (Figure 5) or pressure, as well as Zoon vulvitis (Figure 6). They may also result from premalignant lesions or cancer. In this case, it is necessary to differentiate a lichen sclerosus bed with an episode of genital herpes from a premalignant lesion or even cancer (Figure 7). In this case, a biopsy is indicated.5,9
4
Painful Lipshutz ulcer with urinary retention in an 11-year-old adolescent.
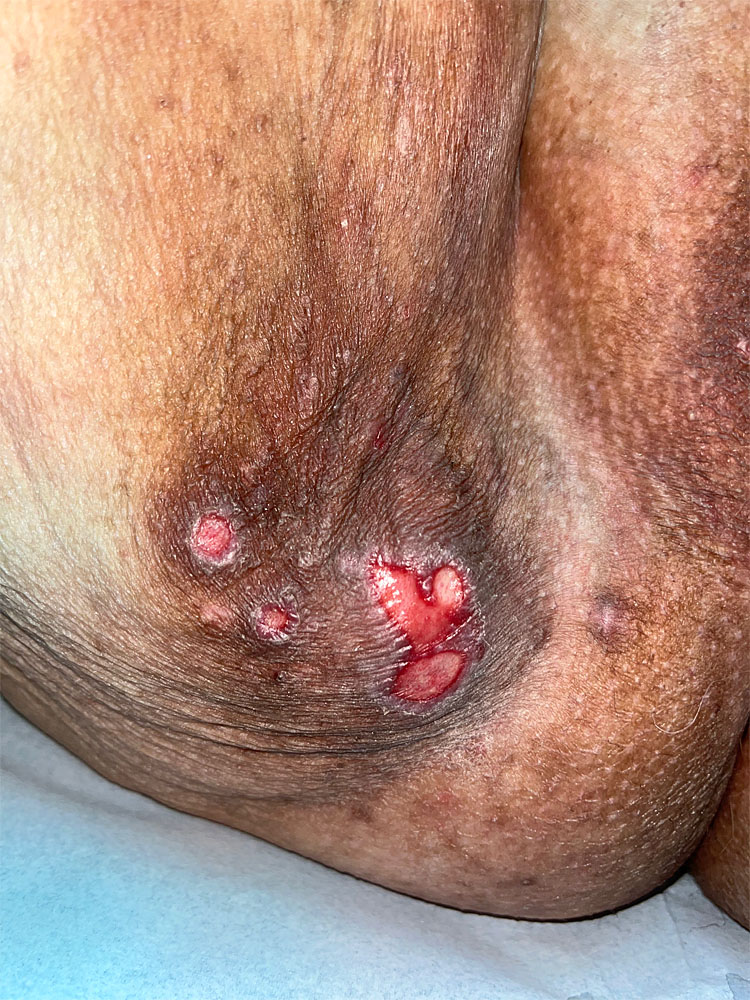
5
Decubitus (compression) ulcer; elderly woman, translator, sitting for more than 12 hours daily.
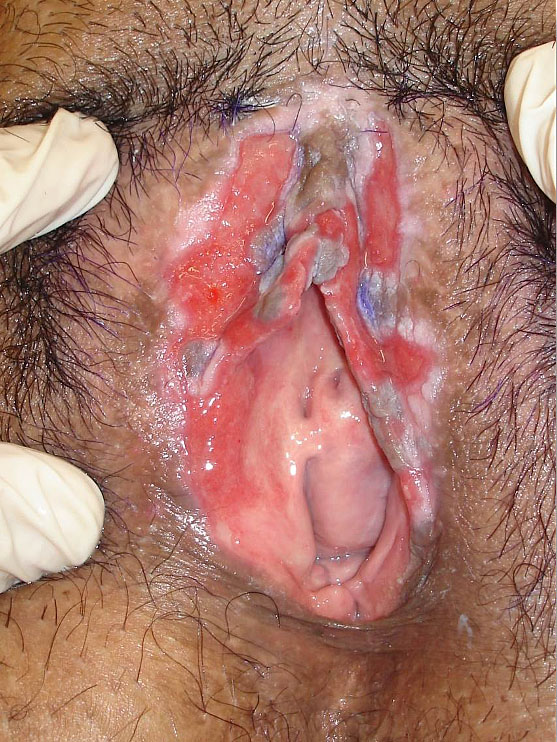
6
Painful ulcer that in the biopsy presented epithelial hyperplasia with ulceration without hypergranulose atypia and dense inflammatory infiltrate with plasmocytic predominance.
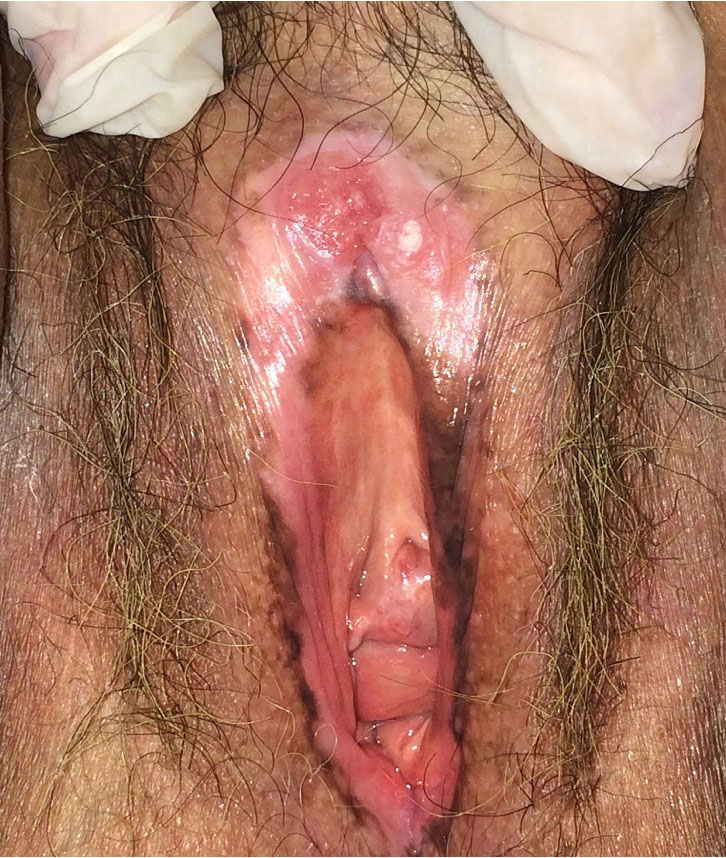
7
Ulcer in a lichen sclerosus bed with biopsy showing vulvar cancer.
In recurrences, the clinical picture is usually milder and non-specific, so the differential diagnosis may include several conditions that mimic the symptoms of genital herpes before medical attention: candidiasis, contact dermatitis (e.g., from topical medications, toilet paper, sanitary pads, soaps, condoms, or menstrual products), trauma from lack of lubrication during intercourse, frequent sexual intercourse, irritation from tight jeans, irritation from a bicycle seat, urinary tract infection, vaginal dryness, razor burns, reactions to hair removal products, hemorrhoids, or anal fissures.1
Diagnosis based on clinical-epidemiological history and physical examination alone is often inaccurate. Therefore, the clinical diagnosis of genital herpes should be confirmed by laboratory tests. It is important to observe that some causes are more common in certain regions.
Special situation
Pregnancy
Genital herpes during pregnancy occurs frequently and is of concern due to the risk of neonatal herpes and its complications.10 The mortality due to neonatal herpes has decreased substantially over the years, but around 20% of survivors have long-term neurologic sequelae, such as chorioretinitis, microcephaly, and skin lesions.11
It is well known that the prevention of neonatal herpes depends both on preventing the acquisition of genital herpes during late pregnancy and avoiding exposure of the neonate to herpetic lesions and viral shedding during delivery.5 The period of acquisition is crucial to understand the risk of transmission. Usually, neonatal herpes is acquired during the intrapartum period.11 The transmission to the neonate from an infected mother can be as high as 30–50% among women who acquire genital herpes near the time of delivery and as low as <1% among women with prenatal histories of recurrent herpes or who acquire genital herpes during the first half of pregnancy.5
As in non-pregnant patients, most pregnant women with new infections are asymptomatic.11,12 The incidence of new HSV-1 or HSV-2 infection during pregnancy is around 2%. Seronegative women who have seropositive partners are at risk for HSV-2 acquisition and the frequency of seroconversion is similar in all three trimesters.12
Recurrent genital HSV in pregnancy is more common if the first episode of herpes occurred during pregnancy (36%), while occurring less frequently in women who experienced the first episode before pregnancy (75%). Some women (14%) have prodromal symptoms or clinical recurrence at delivery.6,11 In the literature, there is no association between herpes infection and miscarriages.6,11
The clinical presentation of genital herpes during pregnancy is similar to that outside of pregnancy.6
Medical and personal history is fundamental to understanding the risk of contamination during pregnancy. The presence of another sexually transmitted infection, a recent relationship, and the partner's history of herpes are important risk factors in acquisition.6
In the case of a suspected genital HSV, it should be confirmed with type-specific laboratory testing. The PCR is three to five times more likely to be positive than cultures12 and should be preferred.6 In scenarios where PCR is not available, it is important to know that primary lesions are more likely than recurrent lesions to yield positive cultures.11
In women with no history of herpes and lesions, during pregnancy, a primary first episode or a non-primary first episode must be distinguished from a recurrence and it can be done by direct detection (PCR or culture) and type-specific serology (IgG).6,13 The presence of antibodies of the same type as the HSV isolated from genital swabs would confirm this episode to be a recurrence rather than a primary infection.13
It is recommended by some authors to test for HIV and other STIs in initial episodes of genital herpes during pregnancy, as it can increase the risk of HIV transmission to the partner and the newborn.6,13
Routine HSV screening is not recommended.5 The HSV screening can be done to identify infected (seropositive) and then offer suppressive antiviral therapy near term. The serology screening can also identify susceptible women (seronegative) whose partners are positive and then talk about strategies to reduce the possibility of new infections during pregnancy.12 The use of condoms should be recommended at least during the third trimester for serodiscordant couples.6
It is important to recognize primary infections during pregnancy and start antiviral prophylaxis in the third trimester to reduce the risks for the newborn. In a study published by Heggarty and others in 2020, they analyzed a questionnaire completed by 354 healthcare providers about genital herpes in pregnancy, and only 68.4% prescribed prophylaxis in this scenario.10
The risk of vertical transmission after a primary outbreak at vaginal delivery is approximately 40–80%.11
The indications for cesarean delivery are as follows: women with active genital lesions or prodromal symptoms (vulvar pain, burning), and when the delivery is expected to occur in less than 6 weeks after the first episode.2,6,13 Cesarean may be offered for women with a primary or non-primary first-episode of genital HSV during the third trimester due to the possibility of prolonged viral shedding.5,11,13
It should be noted that cesarean birth does not completely prevent vertical transmission.5,11
The risks of prematurity should be weighed against the risk of neonatal HSV disease in patients with prelabor rupture of membranes and active genital HSV lesions.11
Women with herpetic lesions on any part of the body must take special care with hand washing to prevent postnatal transmission. Breastfeeding is allowed unless there are breast lesions.11
Immunosuppression
Lesions caused by HSV are common among immunosuppressed patients and are related to the degree of immunosuppression.2 Subclinical shedding can occur frequently.5 These lesions can be atypical, more severe, painful, prolonged, multifocal, coalescing mucocutaneous lesions.2,5 Systemic complications, such as fulminant hepatitis, pneumonia, neurological disease, and disseminated infection have been reported.2
People with a history of genital symptoms may have HSV-2 type-specific serologic testing.5
The synergy between HSV and HIV infection has been reported with the activation of HIV replication and may facilitate its transmission and acquisition.2
Treatment
General population (Table 1)
First clinical episode of genital HSV infection
According to the WHO, 2016, and to the Sexually Transmitted Infections Treatment Guidelines, 2021,5,14 the treatment of herpetic primary infection is recommended for adults and adolescents (strong recommendation, moderate quality evidence).
WHO, 2016, state that acyclovir, valacyclovir, and famcyclovir can be used for a period of 10 days since the symptoms present in the first infection tend to last longer and neurological complications, such as meningitis and urinary retention tend to occur in the last few days of the episode.14 However, the Sexually Transmitted Infections Treatment Guidelines, 2021,5 dictates that the treatment can be extended if healing is incomplete after 10 days of therapy. Although there is no difference in terms of efficacy of these three drugs, acylovir is the most cost-effective, which is why it is the most widely used. Studies compared the use of acyclovir with no treatment and found a reduction in the duration of symptoms, especially pain, viral excretion, and the occurrence of adverse effects caused by the disease. No studies were found comparing valacyclovir or famcyclovir to no treatment.
According to the Sexually Transmitted Infections Treatment Guidelines, 2021,5 acyclovir 200 mg taken orally five times/day is also effective but is not recommended due to the frequency of dosing.
Episodic therapy for recurrent HSV-2 genital herpes
Cases of HSV-2 recurrence are frequent. Viral excretion occurs intermittently even in individuals who remain free of lesions for a long time. Antiviral therapy can be performed only when the disease relapses or in a suppressive manner. The latter has the advantage of lowering the risk of HSV-2 transmission to susceptible partners.
Treatment should be started within the first 24 hours of the onset of symptoms.
Suppressive therapy for recurrent HSV-2 genital herpes
Suppressive treatment is indicated for individuals with frequent recurrences (e.g. 4–6 times a year or more), severe symptoms, or if causing significant distress. In general, the preference is for suppressive rather than episodic treatment, as it promotes a decrease in the frequency of herpetic episodes, in the number of lesions, and in viral shedding, improving the quality of life of these individuals. The length of treatment will be discussed with the patient. There is no need for treatment discontinuation or laboratory monitoring because adverse effects and the occurrence of antiviral resistance due to prolonged treatment are rare.
1
Herpes simplex virus treatment regimens for the general population.
Primary or first-episode infection | Treatment suggested by WHO Guidelines for the Treatment of Genital Herpes Simplex Virus, 201614 and Sexually Transmitted Infections Treatment Guidelines, 20215 | |
Acyclovir 400 mg orally, three times daily, for 7–10 days (and the same thing in the other cells where it applies) OR Acyclovir 200 mg orally, five times daily, for 7–10 days | ||
Valacyclovir 1 g orally, twice daily, for 7–10 days | ||
Famciclovir 250 mg orally, three times/day, for 7–10 days | ||
Symptomatic recurrent episode | Treatment suggested by WHO, Guidelines for the Treatment of Genital Herpes Simplex Virus, 201614 | Treatment suggested by Sexually Transmitted Infections Treatment Guidelines, 20215 |
Acyclovir 400 mg orally, three times daily, for 5 days OR Acyclovir 800 mg orally, twice daily, for 5 days OR Acyclovir 800 mg orally, three times/day, for 2 days | Acyclovir 800 mg orally, twice daily, for 5 days OR Acyclovir 800 mg orally, three times/day, for 2 days | |
Famciclovir 250 mg orally, twice daily, for 5 days | Famciclovir 1 g orally, twice daily, for 1 day OR Famciclovir 500 mg orally once, followed by 250 mg two times/day for 2 days OR Famciclovir 125 mg orally, twice daily, for 5 days | |
Valacyclovir 500 mg orally, twice daily, for 3 days | Valacyclovir 500 mg orally, twice daily, for 3 days OR 1 g orally, once daily, for 5 days | |
Daily suppression | Treatment suggested by WHO, Guidelines for the Treatment of Genital Herpes Simplex Virus, 201614 | Treatment suggested by Sexually Transmitted Infections Treatment Guidelines, 20215 |
Acyclovir 400 mg orally, two times/day | Acyclovir 400 mg orally, two times/day | |
Valacyclovir 500 mg orally, once daily | Valacyclovir 500 mg orally, once daily OR Valacyclovir 1 g orally, once daily | |
Famciclovir 250 mg orally, twice daily | Famciclovir 250 mg orally, twice daily |
Pregnancy
The oral antiviral agents, acyclovir, valacyclovir, and famcyclovir are approved for primary genital herpes, recurrent episodes, and daily suppression, as well as during breastfeeding.6,12 However, according to the CDC, 2021, in analyses of individual antivirals, risk estimates were similar for acyclovir and valacyclovir, although analysis of valacyclovir was based on a limited number of exposed cases, results indicate that it is not a major human teratogen. On the other hand, studies carried out with the use of famcyclovir were based on only 26 exposed pregnancies and should not be viewed with sufficient evidence for use among pregnant women, leaving acyclovir as the first line of treatment because it is the most widely documented antiviral in pregnancy (Table 2).5,13,15
The treatment is recommended to reduce the duration and severity of symptoms and to reduce viral shedding.11,13
Acyclovir is the most well studied in pregnancy. There is no published data on the use of famcyclovir.
Suppressive therapy must begin at 36 weeks of gestation in women with a primary or non-primary first episode, as well as in women with a clinical history of genital herpes. For primary outbreaks that occur in the third trimester, antiviral therapy can be continued until delivery.11,13 For twin pregnancies, the suppressive treatment may begin at 32 weeks.6 This strategy reduces the risk of clinical recurrence of HSV at the time of delivery, cesarean birth for recurrent herpes, and asymptomatic shedding.11
It is important to remember that, due to the higher kidney filtration rate, the doses of antiviral medication used for suppressive therapy for recurrent HSV infection in pregnancy are higher than for non-pregnant women.11,13
2
Herpes simplex virus treatment regimens in pregnant women.
Treatment suggested by ACOG, 202011 | Treatment suggested by Sexually Transmitted Infections Treatment Guidelines, 20215 | ||
Primary or first-episode infection | Acyclovir 400 mg orally, three times daily, for 7–10 days or more until completely healed | Valacyclovir 1 g orally, twice daily, for 7–10 days or more until completely healed | Acyclovir 400 mg orally, three times daily, for 7–10 days or more until completely healed |
Symptomatic recurrent episode | Acyclovir 400 mg orally, three times daily, for 5 days OR 800 mg orally, twice daily, for 5 days | Valacyclovir 500 mg orally, twice daily, for 3 days OR 1 g orally, daily, for 5 days | Acyclovir 800 mg orally two times/day for 5 days OR Acyclovir 800 mg orally three times/day for 2 days |
Daily suppression | Acyclovir 400 mg orally, three times daily, from 36 weeks estimated gestational age until delivery | Valacyclovir 500 mg orally, twice daily, from 36 weeks estimated gestational age until delivery | Acyclovir 400 mg orally three times/day OR Valacyclovir 500 mg orally two times/day |
Severe or disseminated disease | Acyclovir 5–10 mg/kg, intravenously, every 8 hours for 2–7 days, then oral therapy for primary infection to complete 10 days | Acyclovir 5–10 mg/kg, intravenously, every 8 hours until clinical improvement followed by oral antiviral therapy to complete >10 days of total therapy |
Immunosupression
The treatment is the same for people living with HIV, but sometimes the duration may need to be extended for lesion resolution. Suppressive antiviral therapy among HIV positive and HSV infection does not reduce the risk for either HIV or HSV-2 transmission to susceptible sex partners (Table 3).5
In the first episode, the treatment should be given for at least 10 days or until the lesions are completely healed.2
For isolated episodes, it is likely that 5 days of treatment will be effective. For people with an advanced form of the disease, it is possible that therapy may need to be continued beyond this period and the dose may need to be doubled.2
The frequency of clinical recurrences can be reduced by a highly active antiretroviral, but it has less effect on asymptomatic viral shedding.2
People living with HIV usually have a worse response to suppressive antiviral therapy.2
Therapy with acyclovir 5–10 mg/kg IV every 8 hours might be necessary for patients with severe HSV disease in this population.5
People with HIV co-infection are more prone to having resistance to antiherpetic drugs. So if new lesions are still forming after 3–5 days, viral isolation and susceptibility testing should be attempted. Despite this, the clinical response to antiviral therapy is better to guide the treatment instead of the susceptibility testing because of its limited availability. Guidance from a clinical virologist is frequently necessary when clinical resistance is suspected. The systemic therapies foscarnet and cidofovir are options to treat drug resistance.2
3
Herpes simplex virus treatment regimens in immunosuppressed individuals.
Treatment suggested by Sexually Transmitted Infections Treatment Guidelines, 20215 | |
Primary or first-episode infection | Acyclovir† 400 mg orally three times/day for 7–10 days OR Famciclovir 250 mg orally three times/day for 7–10 days OR Valacyclovir 1 g orally two times/day for 7–10 days |
Symptomatic recurrent episode | Acyclovir 400 mg orally three times/day for 5–10 days OR Famciclovir 500 mg orally two times/day for 5–10 days OR Valacyclovir 1 g orally two times/day for 5–10 days |
Daily suppression | Acyclovir 400–800 mg orally two–three times/day OR Famciclovir 500 mg orally two times/day OR Valacyclovir 500 mg orally two times/day |
Severe or disseminated disease | Acyclovir 5–10 mg/kg, intravenously, every 8 hours until clinical improvement followed by oral antiviral therapy to complete >10 days of total therapy |
PRACTICE RECOMMENDATIONS
- HSV is a common sexually transmitted infection worldwide.
- HSV infections are categorized as primary or recurrent, and the clinical manifestations are highly variable.
- Diagnosis is confirmed by PCR and viral type-specific serological tests.
- Treatment can be episodic or suppressive and is not curative.
- Transmission occurs not only by penetrative sexual intercourse, but also by genital skin to genital skin contact with infected partners. These are excreting virus during a clinically evident herpetic episode, but more commonly during an episode of asymptomatic infection.
- The guidance offered to patients by the healthcare provider on HSV prevention is the key to reducing HSV prevalence and decreasing physical and psychosocial distress as well as complications that accompany the HSV virus.
- In pregnancy, suspected genital herpes virus infections should be confirmed with type-specific laboratory testing. However, retesting is not warranted in pregnant women with a history of laboratory-confirmed genital HSV.
- Women with a clinical history of genital herpes should be offered suppressive viral therapy at or beyond 36 weeks of gestation. For primary outbreaks that occur in the third trimester, continuing antiviral therapy until delivery may be considered.
- Because of enhanced renal clearance, the doses of antiviral medication used for suppressive therapy for recurrent HSV infection in pregnancy are higher than the corresponding doses in non-pregnant women.
- Cesarean delivery is indicated in women with active genital lesions or prodromal symptoms, such as vulvar pain or burning at delivery, because these symptoms may indicate viral shedding.
- In the first episode, the treatment should be given for at least 10 days or until the lesions are completely healed in immunosuppressed patients.
- For isolated episodes, it is likely that 5 days of treatment will be effective in HIV patients. For people with advanced disease it is possible that they may need to continue therapy beyond this period and to double the dose.
- Therapy with acyclovir 5–10 mg/kg IV every 8 hours might be necessary for severe HSV disease in HIV population.
CONFLICTS OF INTEREST
The author(s) of this chapter declare that they have no interests that conflict with the contents of the chapter.
Feedback
Publishers’ note: We are constantly trying to update and enhance chapters in this Series. So if you have any constructive comments about this chapter please provide them to us by selecting the "Your Feedback" link in the left-hand column.
REFERENCES
-Garland SM, Steben M. Best Pract Res Clin Obstet Gynaecol 2014;28(7):1098–110. PMID: 25153069. | |
Patel R, Kennedy OJ, Clarke E, et al. 2017 European guidelines for the management of genital herpes. Int J STD AIDS 2017;28(14):1366–79. PMID: 28836892. | |
Cole S. Herpes Simplex Virus Epidemiology, Diagnosis, and Treatment. Nurs Clin N Am 2020;55:337–45. PMID: 32762854. | |
McQuillan G, Kruszon-Moran D, Flagg EW, et al. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief 2018;304:1–8. PMID:29442994. | |
Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep 2021;70(No. RR-4):1–187. PMID: 34292926. | |
Sénat M-V, Anselem O, Picone O, et al. Prevention and management of genital herpes simplex infection during pregnancy and delivery: Guidelines from the French College of Gynaecologists and Obstetricians (CNGOF). Eur J Obstet Gynecol Reprod Biol 2018;224:93–101. PMID: 29571124. | |
Stanaway JD, Wald A, Martin ET, et al. Case-crossover analysis of condom use and HSV-2 acquisition. Sexual Transm Dis 2012;39(5):388. PMID: 22504606. | |
Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis 2009;199:958–64. PMID: 19220143. | |
Workowski KA, Bolan GA. Sexually Transmitted Infections Treatment Guidelines, 2015. MMWR Recomm Rep 2015;64(RR-03):1–137. PMID: 26042815. | |
Heggarty E, Sibiude J, Mandelbrot L, et al. Genital herpes and pregnancy: Evaluating practices and knowledge of French health care providers. Eur J Obstet Gynecol Reprod Biol 2020;249:84–91. PMID: 32122691. | |
ACOG PRACTICE BULLETIN. Clinical Management Guidelines for Obstetrician–Gynecologists. Management of Genital Herpes in Pregnancy Obstetrics & Gynecology 2020;135(5):e193–202. PMID: 32332414. | |
Picone O. Herpès génital et grossesse: épidémiologie, manifestations de la maladie, prévention et dépistage. Recommandations pour la pratique clinique du Collège national des gynécologues obstétriciens français (CNGOF). Gynecol Obstet Fertil Senol 2017;45(12):642–54. PMID: 29146286. | |
British Association for Sexual Health and HIV and the Royal College of Obstetricians and Gynaecologists (RCOG). Management of Genital Herpes in Pregnancy. London: BASHH and RCOG, Guideline date: October 2014 Date of review: by 2018. | |
World Health Organization. Guidelines for the Treatment of Genital Herpes Simplex Virus. Report. Geneva, 2016. (WHO-Guidelines Approved by the Guidelines Review Committee). | |
Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects.JAMA 2010;304(8):859-66. PMID: 20736469 |
Online Study Assessment Option
All readers who are qualified doctors or allied medical professionals can now automatically receive 2 Continuing Professional Development credits from FIGO plus a Study Completion Certificate from GLOWM for successfully answering 4 multiple choice questions (randomly selected) based on the study of this chapter.
Medical students can receive the Study Completion Certificate only.
(To find out more about FIGO’s Continuing Professional Development awards programme CLICK HERE)

